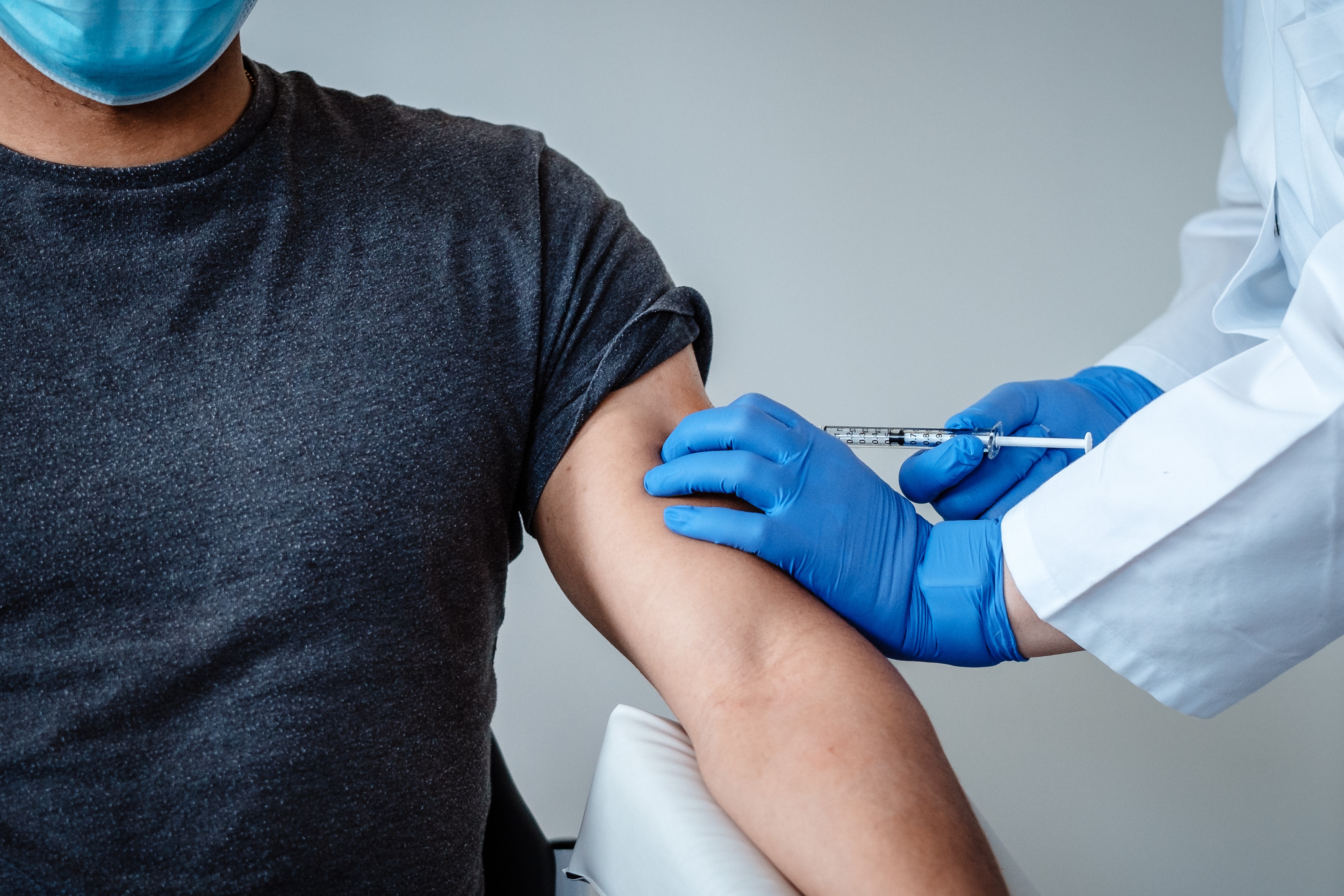

The UK today kicks off its coronavirus vaccination programme after months of uncertainty and over 60,000 deaths attributed to the infection since March.
Front-line NHS staff, care home workers and people over 80s will be the first to get inoculated in the marathon programme which is expected to last well into next year.
Prior to its start, chief executive of NHS England Sir Simon Stevens said: “Tomorrow is the beginning of the biggest vaccination campaign in our history, building on successes from previous campaigns against conditions: diseases like polio, meningitis, and tuberculosis.”
How many people are to be inoculated?
What a great moment – Maggie, the first NHS patient to be vaccinated against COVID-19. A truly historic day here @nhsuhcw and the beginning of the end of this pandemic #ThankYouNHS #CovidVaccine pic.twitter.com/zLWDLTemaT
— NHSEnglandNMD (@NHSEnglandNMD) December 8, 2020
The initial batch includes 800,000 doses of the jab developed by Pfizer and BioNTech, which was approved for emergency use in the UK last week.
Given that each recipient should receive two doses, three weeks apart, the first vaccine batch will cover 400,000 people.
In England 50 hospital hubs have been set up for the inoculation programme, and the jab is also expected to be available at GP surgeries and care homes.
The UK earlier ordered 40 million doses of the Pfizer vaccine, which will be enough to vaccinate 20 million people. By the end of 2020, it is expected to receive a total of four million doses.
The NHS is reportedly in the process of recruiting 30,000 volunteers for different roles, including administering the vaccine.
What other vaccines are in the UK portfolio?
There are six more vaccines that the UK pre-purchased in big amounts, including the homegrown one by AstraZeneca and the University of Oxford.
The UK government currently has deals for a total of 357 million vaccine doses across seven different vaccines developed globally out of over 100, according to its latest statement from 30 November.
The major batch of the pre-ordered doses – 100 million – are those of the AstraZeneca/Oxford University vaccine. Most of them (80 million) are expected to be made in the UK. However, the first 4 million doses of the vaccine which are set to be made available before the end of the year will be imported from the Netherlands and Germany.
The government also secured equal batches – 60 million each – of the jab by US company Novovax, the one by GSK/Sanofi and the one by Valneva. They are all at the different stages of clinical trials and unlikely to be made available until mid-2021. In the case of Novovax and Valneva, some of their vaccines are expected to be manufactured in the UK.
The UK government’s vaccination portfolio also has 30 million doses of the jab by Janssen and 7 million doses of the vaccine by US company Moderna, which can become available in the UK before spring 2021.
How do these vaccines differ?
So far, Pfizer vaccine proved to be the most effective at preventing the illness in its phase 3 trials, with the jab by Moderna showing the 94.5-per cent efficacy.
The Oxford/AstraZeneca vaccine showed to be up to 90 per cent effective and can be stored in standard fridges, which reduces costs.
The Pfizer vaccine appears to be the most logistically challenging as it is required to be kept at ultra-low temperatures (-70 degrees Celsius).
Both Pfizer/BioNTech and Moderna vaccines use a brand-new messenger RNA (mRNA) formula, which might be behind the short-term side effects in fewer than two per cent of recipients, according to scientific magazine Science. They include severe fevers of 39 to 40 degrees Celsius, joint and muscle aches and fatigue among others.
Nature.com pointed out that neither the Pfizer jab nor the AstraZeneca and Moderna ones have demonstrated that they prevent infection altogether or reduce the spread of the virus in a population.
This means that there is a chance that those vaccinated may still transmit the virus to other people who remain vulnerable.
Is there any timeframe for vaccination?
There is no clarity yet.
Downing Street said on Monday that it expects “the majority” of vulnerable people to be vaccinated by the end of winter, however, it did not specify the number of people who will receive the vaccine by March.
It added that the Moderna and AstraZeneca vaccines that are currently being assessed by UK regulators could boost the number of doses available to the public.
NHS England’s national medical director, Prof Stephen Powis earlier warned that the rollout of the vaccine will be “a marathon, not a sprint” and many months will be required to vaccinate anyone who needs it.
Is vaccine hesitancy an issue?
Despite government attempts to promote the Covid vaccination among Britons, there are still concerns about its safety.
About a third of the public said they are unlikely to take the Covid-19 vaccine when it becomes available, according to a recent Opinium poll for the Observer. Key areas of concern mentioned were the vaccine safety and efficacy, with 55 per cent of those polled saying they are worried that it will have side-effects.
Health Secretary Matt Hancock earlier said that he is up for taking the shot live on TV to quell concerns about its safety, noting however that he was not a priority vaccine candidate.
Meanwhile, vaccine hesitancy may be partly related to disinformation narratives that have been actively spread by some opponents of the vaccination on social media.
What other vaccination programmes are there globally?
Many countries seem to be wary of rushing to approve coronavirus vaccines and roll out mass vaccination programmes, with the EU and the US expected to announce their decisions only in the coming weeks.
Among those who have already widely distributed their vaccines are China and Russia, with the latter approving its homegrown vaccine despite Western criticism. Both countries have promoted their vaccines to their allies among developing countries in an apparent bid to exercise soft diplomacy.
Russia began its vaccination programme on 5 December, administering its domestic vaccine Sputnik V to doctors, teachers and social workers in Moscow. The jab is free for Russian citizens and is said to have more than 95 per cent efficacy – without much clinical data available to prove that.
Video of mass vaccination with the #SputnikV vaccine against #COVID-19 in Moscow by @SputnikInt news agency. pic.twitter.com/IuIj3OckdH
— Sputnik V (@sputnikvaccine) December 5, 2020
In case of China, it has not so far launched a full-blown vaccination campaign but it did inject almost a million people with its emergency vaccine back in July, including government officials, students and workers travelling overseas.
It said that those injected travelled to more than 150 countries and “there has not been a single case of infection after inoculation”, China National Pharmaceutical Group, or Sinopharm, which developed the vaccine said in a statement.
